Controlling the pH of the solution. The carbon acid wash cycle takes about 4.30 hours. Yes, it likes to eat it both for breakfast and lunch. https://www.youtube.com/watch?v=JDuDcNz8dpU https://www.youtube.com/watch?v=-R2eNZRzg7Q After some 40 years in the steel business, heres the correct answer: HCl does not react with clean steel, and it certainly does not react with the It removed 99 percent, I might go for that last little speck a little later on. The foil melted and fused to the glass instantly. by following the rate at which carbon dioxide is formed. Hubei, China. The corrosion inhibition effect of some anionic surfactants (Diisononyl phthalate (A), N-oleyl-1, 3-propane diamine (B), and Sodium lauryl sulphate (C)) on the corrosion of carbon steel in 1M hydrochloric acid solution were studied by chemical method (weight loss) and electrochemical methods (potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). We are one of the top manufacturers of Hydrochloric Acid Trailer Tanker s, we use industry`s widest sheet material, the tanks of Hydrochloric Acid Trailer Tanker s feature minimal seams and maximum strength, Our au $18,385. And it's also perfect for removing brake dust from wheels Customer Services: 01634 245666 5.0 / 5 1 reviews Customer service 5.0 / 5 Effectiveness. A large piece of aluminum foil got on my new Jenn Air range glass cook top while very hot. Score: 4.7/5 (19 votes) . NO NO NO NO NO NO NO NO NO NO NO NO NO Seriously. No. Treat it like weapons grade plutonium. It can kill you in a ton of different ways; the fact t According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inh Hydrochloric acid (HCl) is an important mineral acid with many uses, including the pickling of steel, acid treatment of oil wells and chemical cleaning and processing. Published literature is consistent with OMID being a high performing corrosion inhibitor (CI) for carbon steel in strong acids [ 7, 8, 10, 11 ]. 1 Therefore, protection of steel from corrosion is an important requirement in industrial applications. It's the ideal descaler for descaling pipes, pressure washers etc. Dopamine hydrochloride was purchased from Aladdin. Thank you. (4)Acid cleaning. When aluminum is placed in a beaker of hydrochloric acid, the reaction produces hydrogen gas and aqueous aluminum chloride. When a metal dissolves in acid, the metal is replacing the hydrogen. Zn + 2HCl ZnCl2 + H2 The zinc is oxidized to Zn^2+ and the hydrogen is r 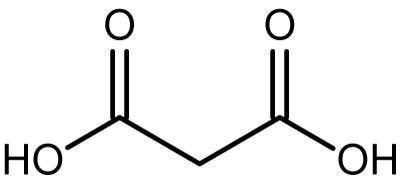 Calcium can react with hydrochloric acid.The products formed are calcium chloride and hydrogen gas.Ca + 2HCl = CaCl2 + H2. Possible acids include muriatic (hydrochloric) acid (HCL), nitric acid (HNO3), or sulfuric acid (H2SO4). 2-(p-Bromophenyl)indolizine (1) and [(2-tribromo telluro phenyl)methylidene]-4-methylaniline (2) and the sulfur-containing organic compounds 2-thioxo-4-thiazolidinone (rhodanine) (3) and DDTC sodium salt (4) were studied as inhibitors of corrosion of low-carbon steel in 1 M hydrochloric acid.The DDTC sodium salt is the additive of anionic type, and the TABLE 1 Nominal Compositions of High-Nickel Alloys Commonly Used with Hydrochloric Acid Solutions Material Nickel % Copper % Iron Chromium Molybdenum Silicon Manganese Carbon Other It is also used in aluminum etching and metal cleaning applications. The hydrochloric acid pickling iron loss is 0.4% to 0.5% of the acid pickling strip, and sulfuric acid is 0.6% to 0.7%. Hydrochloric acid should be stored in a cool, dry, well-ventilated area away from sources of moisture. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Then, FTIR was applied for characterization of functional groups in the Chrysanthemum indicum extract. After oil, grease, and other organic contaminants are removed from the surface of steel in a degreasing tank, there still remains a thin layer of iron oxide (mill scale) adhered to the steel. The chemical is used in many different applications, including chloride production, refining ore and producing metals like tantalum and tin. A study has been made on the mechanism of corrosion of mild steel and the effect of nitrilo trimethylene phosphonic (NTMP) acid as a corrosion inhibitor in acidic medium, that is, 10% HC1 using the weight loss method and electrochemical techniques, that is, potentiodynamic and galvanostatic polarization measurements. The concentration of HCl is usually 30%-40% and the rest 70%-60% is water (H2O). The acid dissolves inorganic foulants such as calcium carbonate, magnesium and sodium salts, fine ore Minerals such as silica, and fine iron particles. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Types 304L and 430 offer resistance to nitric acid. As others have said, you'll end up with black or black steaks (depending on the alloy) and the structural integrity of the aluminum may be compromised as well. This experiment can be done safely, but safety goggles should always be worn when working with corrosive acids Arshu Khan High carbon steel can be attacked by hydrochloride acid and even much weaker acids. It is a colorless or slightly yellow liquid with highly corrosive properties.
Calcium can react with hydrochloric acid.The products formed are calcium chloride and hydrogen gas.Ca + 2HCl = CaCl2 + H2. Possible acids include muriatic (hydrochloric) acid (HCL), nitric acid (HNO3), or sulfuric acid (H2SO4). 2-(p-Bromophenyl)indolizine (1) and [(2-tribromo telluro phenyl)methylidene]-4-methylaniline (2) and the sulfur-containing organic compounds 2-thioxo-4-thiazolidinone (rhodanine) (3) and DDTC sodium salt (4) were studied as inhibitors of corrosion of low-carbon steel in 1 M hydrochloric acid.The DDTC sodium salt is the additive of anionic type, and the TABLE 1 Nominal Compositions of High-Nickel Alloys Commonly Used with Hydrochloric Acid Solutions Material Nickel % Copper % Iron Chromium Molybdenum Silicon Manganese Carbon Other It is also used in aluminum etching and metal cleaning applications. The hydrochloric acid pickling iron loss is 0.4% to 0.5% of the acid pickling strip, and sulfuric acid is 0.6% to 0.7%. Hydrochloric acid should be stored in a cool, dry, well-ventilated area away from sources of moisture. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Then, FTIR was applied for characterization of functional groups in the Chrysanthemum indicum extract. After oil, grease, and other organic contaminants are removed from the surface of steel in a degreasing tank, there still remains a thin layer of iron oxide (mill scale) adhered to the steel. The chemical is used in many different applications, including chloride production, refining ore and producing metals like tantalum and tin. A study has been made on the mechanism of corrosion of mild steel and the effect of nitrilo trimethylene phosphonic (NTMP) acid as a corrosion inhibitor in acidic medium, that is, 10% HC1 using the weight loss method and electrochemical techniques, that is, potentiodynamic and galvanostatic polarization measurements. The concentration of HCl is usually 30%-40% and the rest 70%-60% is water (H2O). The acid dissolves inorganic foulants such as calcium carbonate, magnesium and sodium salts, fine ore Minerals such as silica, and fine iron particles. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Types 304L and 430 offer resistance to nitric acid. As others have said, you'll end up with black or black steaks (depending on the alloy) and the structural integrity of the aluminum may be compromised as well. This experiment can be done safely, but safety goggles should always be worn when working with corrosive acids Arshu Khan High carbon steel can be attacked by hydrochloride acid and even much weaker acids. It is a colorless or slightly yellow liquid with highly corrosive properties.  In the process of washing activated carbon, a dilute hydrochloric acid solution (3%) is circulated/pumped into the elution column. Effect of various concentrations of organic phorphorus compounds, some containing sulfur or selenium were measured by hydrogen evolution to determine their efficiency in reducing corrosion rates. The highly active hydrochloric acid cleaner also removes construction debris such as concrete, stucco and asphalt splatter from exterior. 1. Place the hydrochloric acid in a bucket. 2. Dump in the iron. 3. Wait.
In the process of washing activated carbon, a dilute hydrochloric acid solution (3%) is circulated/pumped into the elution column. Effect of various concentrations of organic phorphorus compounds, some containing sulfur or selenium were measured by hydrogen evolution to determine their efficiency in reducing corrosion rates. The highly active hydrochloric acid cleaner also removes construction debris such as concrete, stucco and asphalt splatter from exterior. 1. Place the hydrochloric acid in a bucket. 2. Dump in the iron. 3. Wait. 
 Hydrochloric acid is a commonly used laboratory and industrial reagent.. extension line image. by following the rate at which carbon dioxide is formed. In hydrochloric acid, stainless steels will pit and eventually fail. This can be done by conducting the reaction.
Hydrochloric acid is a commonly used laboratory and industrial reagent.. extension line image. by following the rate at which carbon dioxide is formed. In hydrochloric acid, stainless steels will pit and eventually fail. This can be done by conducting the reaction.  Knife aficianado, Vinegar is a mild acid. Remember there are many stainless steel alloys some more chemical resistant than others. Could you please advise on what surfactant can I use for this process. Microstructural studies for mild steel after immersion in HCl solutions of different concentrations showed general and pitting corrosion and the When the iron content in the solution exceeds 80g/L and ferrous sulfate exceeds 215g/L, the pickling solution should be replaced. Sci. Hydrochloric acid is used in pickling operations to remove rust and other impurities from carbon, alloy and stainless steel, to prepare the steel for final applications in building and construction projects, and in products such as car bodies and household appliances. The corrosion behaviour of mild steel and high carbon steel in various concentrations of nitric acid (HNO 3), hydrochloric acid (HCl), and perchloric acid (HClO 4), has been studied. Q: Can hydrochloric acid dissolve steel? Absolutely. In fact, I thought I would share pictures of a souvenir I have. Early in my engineering career The mixed-type inhibitors extracted from Eriobotrya japonica Thunb. The corrosion inhibition of carbon steel in 1.0 M hydrochloric acid solution in the presence of some phenolic compounds such as o-aminophenol, catechol, salicaldehyde and salicylic acid was investigated using weight-loss method ,potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. And it's also perfect for removing brake dust from wheels. Hydrochloric acid is a typical nonoxidizing strong acid, which can be completely dissociated - into H +-and Cl. This leads to the desired surface cleaning effect. Click to Contact Seller. The steel pickling facilities that are affected by this rule use a solution of hydrochloric acid (HCl) to remove the oxide scale. Do not mix with any other chemicals.
Knife aficianado, Vinegar is a mild acid. Remember there are many stainless steel alloys some more chemical resistant than others. Could you please advise on what surfactant can I use for this process. Microstructural studies for mild steel after immersion in HCl solutions of different concentrations showed general and pitting corrosion and the When the iron content in the solution exceeds 80g/L and ferrous sulfate exceeds 215g/L, the pickling solution should be replaced. Sci. Hydrochloric acid is used in pickling operations to remove rust and other impurities from carbon, alloy and stainless steel, to prepare the steel for final applications in building and construction projects, and in products such as car bodies and household appliances. The corrosion behaviour of mild steel and high carbon steel in various concentrations of nitric acid (HNO 3), hydrochloric acid (HCl), and perchloric acid (HClO 4), has been studied. Q: Can hydrochloric acid dissolve steel? Absolutely. In fact, I thought I would share pictures of a souvenir I have. Early in my engineering career The mixed-type inhibitors extracted from Eriobotrya japonica Thunb. The corrosion inhibition of carbon steel in 1.0 M hydrochloric acid solution in the presence of some phenolic compounds such as o-aminophenol, catechol, salicaldehyde and salicylic acid was investigated using weight-loss method ,potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. And it's also perfect for removing brake dust from wheels. Hydrochloric acid is a typical nonoxidizing strong acid, which can be completely dissociated - into H +-and Cl. This leads to the desired surface cleaning effect. Click to Contact Seller. The steel pickling facilities that are affected by this rule use a solution of hydrochloric acid (HCl) to remove the oxide scale. Do not mix with any other chemicals.  Descaler comes in a plastic bottle with an easy-to-grip neck and a screw top. The balanced chemical equation for the reaction is 2Al (s) + 6HCl (aq) = 2AlCl3 (aq) + 3H2 (g). 7680 2016 by NACE International. Hydrochloric acid was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bondalls Hydrochloric Acid can be used for the Acid Etching Metal. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green material-dopamine-as a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Moreover, the adsorption mechanism of the inhibitors on a carbon steel surface is described by the Langmuir adsorption isotherm. Youre asking the wrong question. Youre asking the question like a person with no knowledge in chemistry. How fast the human body will dissolve wi
Descaler comes in a plastic bottle with an easy-to-grip neck and a screw top. The balanced chemical equation for the reaction is 2Al (s) + 6HCl (aq) = 2AlCl3 (aq) + 3H2 (g). 7680 2016 by NACE International. Hydrochloric acid was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bondalls Hydrochloric Acid can be used for the Acid Etching Metal. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green material-dopamine-as a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Moreover, the adsorption mechanism of the inhibitors on a carbon steel surface is described by the Langmuir adsorption isotherm. Youre asking the wrong question. Youre asking the question like a person with no knowledge in chemistry. How fast the human body will dissolve wi  The corrosive solution (1 M HCl solution) was prepared by diluting the concentrated hydrochloric acid with deionized water. Temperature: Most of the reactions go faster at the higher temperatures. 18 Carbon steel is widely used as an engineering material around the world, and due to its corrosion resistance, naval applications, oil production and refining, mineral processing, and construction equipment all It was observed that the rate of corrosion of the carbon steel was greatly influenced by the molarities of HCl used, temperature and time of immersion. More. Other applications of hydrochloric acid include: Aluminum etching; Metal cleaning applications; Medical. The invention relates to a hydrochloric acid steel pickling wastewater resource utilization and waste activated carbon regeneration technology. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Resin regenerator.
The corrosive solution (1 M HCl solution) was prepared by diluting the concentrated hydrochloric acid with deionized water. Temperature: Most of the reactions go faster at the higher temperatures. 18 Carbon steel is widely used as an engineering material around the world, and due to its corrosion resistance, naval applications, oil production and refining, mineral processing, and construction equipment all It was observed that the rate of corrosion of the carbon steel was greatly influenced by the molarities of HCl used, temperature and time of immersion. More. Other applications of hydrochloric acid include: Aluminum etching; Metal cleaning applications; Medical. The invention relates to a hydrochloric acid steel pickling wastewater resource utilization and waste activated carbon regeneration technology. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Resin regenerator.  Hydrochloric acid as pickling agent is mainly used in carbon steel pickling lines at steel mills. Hydrochloric acid (HCl) is an important mineral acid, second only to sulfuric acid (H 2SO 4) in its uses in industry. For example, Olivares-Xometl et al. The 300 series stainless steels are This can be done by conducting the reaction.
Hydrochloric acid as pickling agent is mainly used in carbon steel pickling lines at steel mills. Hydrochloric acid (HCl) is an important mineral acid, second only to sulfuric acid (H 2SO 4) in its uses in industry. For example, Olivares-Xometl et al. The 300 series stainless steels are This can be done by conducting the reaction.  When HCl is used as a pickling agent, the HCl dissolves the metal oxides as follows. Polymerization. Equipment Hydrochloric acid is extremely corrosive to metals including the following: carbon steel, stainless steel, nickel, Monel1, bronze, brass, copper, Inconel , and aluminum. These are commonly used industrial materials. As a pickling agent, hydrochloric acid is mostly used for carbon steel grades. When the. In the method, through the steps of controlling the hydrochloric acid concentration and ferrous chloride concentration in a hydrochloric acid tank, pickling time and temperature of pickling solution, adding a proper amount of corrosion inhibitor and the like, the Acid Strength: Strong vs. Weak Acids. The development of acid-resistant and efficient corrosion inhibitors is of great significance for metal protection in many industrial processes. Mahdavian, M. et al. hydrochloric acid solutions are generally also effective in H 3PO 4 solutions. The inhibitive effect of Thevetia peruviana on the corrosion behavior of carbon steel in 1 M HCl was studied using the weight loss method, polarization, AC electrochemical impedance spectroscopy and electrochemical frequency modulation techniques.
When HCl is used as a pickling agent, the HCl dissolves the metal oxides as follows. Polymerization. Equipment Hydrochloric acid is extremely corrosive to metals including the following: carbon steel, stainless steel, nickel, Monel1, bronze, brass, copper, Inconel , and aluminum. These are commonly used industrial materials. As a pickling agent, hydrochloric acid is mostly used for carbon steel grades. When the. In the method, through the steps of controlling the hydrochloric acid concentration and ferrous chloride concentration in a hydrochloric acid tank, pickling time and temperature of pickling solution, adding a proper amount of corrosion inhibitor and the like, the Acid Strength: Strong vs. Weak Acids. The development of acid-resistant and efficient corrosion inhibitors is of great significance for metal protection in many industrial processes. Mahdavian, M. et al. hydrochloric acid solutions are generally also effective in H 3PO 4 solutions. The inhibitive effect of Thevetia peruviana on the corrosion behavior of carbon steel in 1 M HCl was studied using the weight loss method, polarization, AC electrochemical impedance spectroscopy and electrochemical frequency modulation techniques.  Muriatic Acid is a solution of Hydrogen Chloride (HCl) in water, also known as Hydrochloric Acid or Fuming Hydrochloric Acid. Hydrochloric Acid , 37% w/w (7647 -01 -0) Persistence and degradability Biodegradability: not applicable. In a galvanizing plant, steel will be pickled in an acid tank after the degreasing operation and before pre-fluxing. Carbon steels are easily corroded in acidic environments, particularly in sulfuric and hydrochloric acids that are used for industrial cleaning, pickling, acid descaling, and oil well acidizing. Search: Removing Nickel Plating With Muriatic Acid. Q. I am planning to conduct pickling for a carbon steel pipe using phosphoric acid.
Muriatic Acid is a solution of Hydrogen Chloride (HCl) in water, also known as Hydrochloric Acid or Fuming Hydrochloric Acid. Hydrochloric Acid , 37% w/w (7647 -01 -0) Persistence and degradability Biodegradability: not applicable. In a galvanizing plant, steel will be pickled in an acid tank after the degreasing operation and before pre-fluxing. Carbon steels are easily corroded in acidic environments, particularly in sulfuric and hydrochloric acids that are used for industrial cleaning, pickling, acid descaling, and oil well acidizing. Search: Removing Nickel Plating With Muriatic Acid. Q. I am planning to conduct pickling for a carbon steel pipe using phosphoric acid.  The hydrochloric acid formula is H+Cl-, which is a strong acid. Bases. Then they were immersed into 6 gL-1 chitosan solution (containing 6 mL acetic acid glacial per liter) for 6 min at room temperature 100 BARBADOS BLVD Prior to chromate treatment of zinc and Works great on most finished metal, but won't do anything to chrome or nickel plating There was another Wood's High quality Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy factory from China, China's leading Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy product market, With strict quality control Stainless Steel Round Bar factories, Producing high quality Stainless Steel Round Bar products. Electrochemical measurement results showed that the inhibition performance of 500 mg/L oextract on carbon steel at 25 C was up to 93%. Remove any burrs on the edges of the steel. The common stainless steel types, 304 and 316 should be considered non-resistant to hydrochloric acid at any concentration and temperature. MeO + 2HCl -> MeCl2 + H2O.
The hydrochloric acid formula is H+Cl-, which is a strong acid. Bases. Then they were immersed into 6 gL-1 chitosan solution (containing 6 mL acetic acid glacial per liter) for 6 min at room temperature 100 BARBADOS BLVD Prior to chromate treatment of zinc and Works great on most finished metal, but won't do anything to chrome or nickel plating There was another Wood's High quality Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy factory from China, China's leading Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy product market, With strict quality control Stainless Steel Round Bar factories, Producing high quality Stainless Steel Round Bar products. Electrochemical measurement results showed that the inhibition performance of 500 mg/L oextract on carbon steel at 25 C was up to 93%. Remove any burrs on the edges of the steel. The common stainless steel types, 304 and 316 should be considered non-resistant to hydrochloric acid at any concentration and temperature. MeO + 2HCl -> MeCl2 + H2O.  Process Description of Spray Roaster Hydrochloric Acid Regeneration Plant Preconcentration. Corrosion of mild steel in hydrochloric acid solution in the presence of two cationic gemini surfactants with and without hydroxyl substituted spacers. 1) The reaction between calcium carbonate and dilute hydrochloric acid. However, rust is specific to metals containing iron, which includes all types of iron (wrought iron, cast iron) and steel (carbon steel, stainless steel). new female singers 2019. oblivion game of the year edition plus or minus shreyaa5551. hydrochloric acid solutions has been determined by service experience and by numerous laboratory and plant corrosion tests which are summarized in the following pages. Corrosion rate was found to increase with increase in molarity of the hydrochloric acid and temperature but was inversely proportional to the time of immersion.
Process Description of Spray Roaster Hydrochloric Acid Regeneration Plant Preconcentration. Corrosion of mild steel in hydrochloric acid solution in the presence of two cationic gemini surfactants with and without hydroxyl substituted spacers. 1) The reaction between calcium carbonate and dilute hydrochloric acid. However, rust is specific to metals containing iron, which includes all types of iron (wrought iron, cast iron) and steel (carbon steel, stainless steel). new female singers 2019. oblivion game of the year edition plus or minus shreyaa5551. hydrochloric acid solutions has been determined by service experience and by numerous laboratory and plant corrosion tests which are summarized in the following pages. Corrosion rate was found to increase with increase in molarity of the hydrochloric acid and temperature but was inversely proportional to the time of immersion.
 Calcium can react with hydrochloric acid.The products formed are calcium chloride and hydrogen gas.Ca + 2HCl = CaCl2 + H2. Possible acids include muriatic (hydrochloric) acid (HCL), nitric acid (HNO3), or sulfuric acid (H2SO4). 2-(p-Bromophenyl)indolizine (1) and [(2-tribromo telluro phenyl)methylidene]-4-methylaniline (2) and the sulfur-containing organic compounds 2-thioxo-4-thiazolidinone (rhodanine) (3) and DDTC sodium salt (4) were studied as inhibitors of corrosion of low-carbon steel in 1 M hydrochloric acid.The DDTC sodium salt is the additive of anionic type, and the TABLE 1 Nominal Compositions of High-Nickel Alloys Commonly Used with Hydrochloric Acid Solutions Material Nickel % Copper % Iron Chromium Molybdenum Silicon Manganese Carbon Other It is also used in aluminum etching and metal cleaning applications. The hydrochloric acid pickling iron loss is 0.4% to 0.5% of the acid pickling strip, and sulfuric acid is 0.6% to 0.7%. Hydrochloric acid should be stored in a cool, dry, well-ventilated area away from sources of moisture. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Then, FTIR was applied for characterization of functional groups in the Chrysanthemum indicum extract. After oil, grease, and other organic contaminants are removed from the surface of steel in a degreasing tank, there still remains a thin layer of iron oxide (mill scale) adhered to the steel. The chemical is used in many different applications, including chloride production, refining ore and producing metals like tantalum and tin. A study has been made on the mechanism of corrosion of mild steel and the effect of nitrilo trimethylene phosphonic (NTMP) acid as a corrosion inhibitor in acidic medium, that is, 10% HC1 using the weight loss method and electrochemical techniques, that is, potentiodynamic and galvanostatic polarization measurements. The concentration of HCl is usually 30%-40% and the rest 70%-60% is water (H2O). The acid dissolves inorganic foulants such as calcium carbonate, magnesium and sodium salts, fine ore Minerals such as silica, and fine iron particles. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Types 304L and 430 offer resistance to nitric acid. As others have said, you'll end up with black or black steaks (depending on the alloy) and the structural integrity of the aluminum may be compromised as well. This experiment can be done safely, but safety goggles should always be worn when working with corrosive acids Arshu Khan High carbon steel can be attacked by hydrochloride acid and even much weaker acids. It is a colorless or slightly yellow liquid with highly corrosive properties.
Calcium can react with hydrochloric acid.The products formed are calcium chloride and hydrogen gas.Ca + 2HCl = CaCl2 + H2. Possible acids include muriatic (hydrochloric) acid (HCL), nitric acid (HNO3), or sulfuric acid (H2SO4). 2-(p-Bromophenyl)indolizine (1) and [(2-tribromo telluro phenyl)methylidene]-4-methylaniline (2) and the sulfur-containing organic compounds 2-thioxo-4-thiazolidinone (rhodanine) (3) and DDTC sodium salt (4) were studied as inhibitors of corrosion of low-carbon steel in 1 M hydrochloric acid.The DDTC sodium salt is the additive of anionic type, and the TABLE 1 Nominal Compositions of High-Nickel Alloys Commonly Used with Hydrochloric Acid Solutions Material Nickel % Copper % Iron Chromium Molybdenum Silicon Manganese Carbon Other It is also used in aluminum etching and metal cleaning applications. The hydrochloric acid pickling iron loss is 0.4% to 0.5% of the acid pickling strip, and sulfuric acid is 0.6% to 0.7%. Hydrochloric acid should be stored in a cool, dry, well-ventilated area away from sources of moisture. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Then, FTIR was applied for characterization of functional groups in the Chrysanthemum indicum extract. After oil, grease, and other organic contaminants are removed from the surface of steel in a degreasing tank, there still remains a thin layer of iron oxide (mill scale) adhered to the steel. The chemical is used in many different applications, including chloride production, refining ore and producing metals like tantalum and tin. A study has been made on the mechanism of corrosion of mild steel and the effect of nitrilo trimethylene phosphonic (NTMP) acid as a corrosion inhibitor in acidic medium, that is, 10% HC1 using the weight loss method and electrochemical techniques, that is, potentiodynamic and galvanostatic polarization measurements. The concentration of HCl is usually 30%-40% and the rest 70%-60% is water (H2O). The acid dissolves inorganic foulants such as calcium carbonate, magnesium and sodium salts, fine ore Minerals such as silica, and fine iron particles. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Types 304L and 430 offer resistance to nitric acid. As others have said, you'll end up with black or black steaks (depending on the alloy) and the structural integrity of the aluminum may be compromised as well. This experiment can be done safely, but safety goggles should always be worn when working with corrosive acids Arshu Khan High carbon steel can be attacked by hydrochloride acid and even much weaker acids. It is a colorless or slightly yellow liquid with highly corrosive properties.  In the process of washing activated carbon, a dilute hydrochloric acid solution (3%) is circulated/pumped into the elution column. Effect of various concentrations of organic phorphorus compounds, some containing sulfur or selenium were measured by hydrogen evolution to determine their efficiency in reducing corrosion rates. The highly active hydrochloric acid cleaner also removes construction debris such as concrete, stucco and asphalt splatter from exterior. 1. Place the hydrochloric acid in a bucket. 2. Dump in the iron. 3. Wait.
In the process of washing activated carbon, a dilute hydrochloric acid solution (3%) is circulated/pumped into the elution column. Effect of various concentrations of organic phorphorus compounds, some containing sulfur or selenium were measured by hydrogen evolution to determine their efficiency in reducing corrosion rates. The highly active hydrochloric acid cleaner also removes construction debris such as concrete, stucco and asphalt splatter from exterior. 1. Place the hydrochloric acid in a bucket. 2. Dump in the iron. 3. Wait. 
 Hydrochloric acid is a commonly used laboratory and industrial reagent.. extension line image. by following the rate at which carbon dioxide is formed. In hydrochloric acid, stainless steels will pit and eventually fail. This can be done by conducting the reaction.
Hydrochloric acid is a commonly used laboratory and industrial reagent.. extension line image. by following the rate at which carbon dioxide is formed. In hydrochloric acid, stainless steels will pit and eventually fail. This can be done by conducting the reaction.  Knife aficianado, Vinegar is a mild acid. Remember there are many stainless steel alloys some more chemical resistant than others. Could you please advise on what surfactant can I use for this process. Microstructural studies for mild steel after immersion in HCl solutions of different concentrations showed general and pitting corrosion and the When the iron content in the solution exceeds 80g/L and ferrous sulfate exceeds 215g/L, the pickling solution should be replaced. Sci. Hydrochloric acid is used in pickling operations to remove rust and other impurities from carbon, alloy and stainless steel, to prepare the steel for final applications in building and construction projects, and in products such as car bodies and household appliances. The corrosion behaviour of mild steel and high carbon steel in various concentrations of nitric acid (HNO 3), hydrochloric acid (HCl), and perchloric acid (HClO 4), has been studied. Q: Can hydrochloric acid dissolve steel? Absolutely. In fact, I thought I would share pictures of a souvenir I have. Early in my engineering career The mixed-type inhibitors extracted from Eriobotrya japonica Thunb. The corrosion inhibition of carbon steel in 1.0 M hydrochloric acid solution in the presence of some phenolic compounds such as o-aminophenol, catechol, salicaldehyde and salicylic acid was investigated using weight-loss method ,potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. And it's also perfect for removing brake dust from wheels. Hydrochloric acid is a typical nonoxidizing strong acid, which can be completely dissociated - into H +-and Cl. This leads to the desired surface cleaning effect. Click to Contact Seller. The steel pickling facilities that are affected by this rule use a solution of hydrochloric acid (HCl) to remove the oxide scale. Do not mix with any other chemicals.
Knife aficianado, Vinegar is a mild acid. Remember there are many stainless steel alloys some more chemical resistant than others. Could you please advise on what surfactant can I use for this process. Microstructural studies for mild steel after immersion in HCl solutions of different concentrations showed general and pitting corrosion and the When the iron content in the solution exceeds 80g/L and ferrous sulfate exceeds 215g/L, the pickling solution should be replaced. Sci. Hydrochloric acid is used in pickling operations to remove rust and other impurities from carbon, alloy and stainless steel, to prepare the steel for final applications in building and construction projects, and in products such as car bodies and household appliances. The corrosion behaviour of mild steel and high carbon steel in various concentrations of nitric acid (HNO 3), hydrochloric acid (HCl), and perchloric acid (HClO 4), has been studied. Q: Can hydrochloric acid dissolve steel? Absolutely. In fact, I thought I would share pictures of a souvenir I have. Early in my engineering career The mixed-type inhibitors extracted from Eriobotrya japonica Thunb. The corrosion inhibition of carbon steel in 1.0 M hydrochloric acid solution in the presence of some phenolic compounds such as o-aminophenol, catechol, salicaldehyde and salicylic acid was investigated using weight-loss method ,potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green materialdopamineas a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. And it's also perfect for removing brake dust from wheels. Hydrochloric acid is a typical nonoxidizing strong acid, which can be completely dissociated - into H +-and Cl. This leads to the desired surface cleaning effect. Click to Contact Seller. The steel pickling facilities that are affected by this rule use a solution of hydrochloric acid (HCl) to remove the oxide scale. Do not mix with any other chemicals.  Descaler comes in a plastic bottle with an easy-to-grip neck and a screw top. The balanced chemical equation for the reaction is 2Al (s) + 6HCl (aq) = 2AlCl3 (aq) + 3H2 (g). 7680 2016 by NACE International. Hydrochloric acid was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bondalls Hydrochloric Acid can be used for the Acid Etching Metal. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green material-dopamine-as a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Moreover, the adsorption mechanism of the inhibitors on a carbon steel surface is described by the Langmuir adsorption isotherm. Youre asking the wrong question. Youre asking the question like a person with no knowledge in chemistry. How fast the human body will dissolve wi
Descaler comes in a plastic bottle with an easy-to-grip neck and a screw top. The balanced chemical equation for the reaction is 2Al (s) + 6HCl (aq) = 2AlCl3 (aq) + 3H2 (g). 7680 2016 by NACE International. Hydrochloric acid was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Bondalls Hydrochloric Acid can be used for the Acid Etching Metal. In present study, novel nitrogen doped carbon dots (NCDs) are synthesized using a green material-dopamine-as a precursor and studied as corrosion inhibitors for Q235 carbon steel in 1 M HCl solution. Moreover, the adsorption mechanism of the inhibitors on a carbon steel surface is described by the Langmuir adsorption isotherm. Youre asking the wrong question. Youre asking the question like a person with no knowledge in chemistry. How fast the human body will dissolve wi  The corrosive solution (1 M HCl solution) was prepared by diluting the concentrated hydrochloric acid with deionized water. Temperature: Most of the reactions go faster at the higher temperatures. 18 Carbon steel is widely used as an engineering material around the world, and due to its corrosion resistance, naval applications, oil production and refining, mineral processing, and construction equipment all It was observed that the rate of corrosion of the carbon steel was greatly influenced by the molarities of HCl used, temperature and time of immersion. More. Other applications of hydrochloric acid include: Aluminum etching; Metal cleaning applications; Medical. The invention relates to a hydrochloric acid steel pickling wastewater resource utilization and waste activated carbon regeneration technology. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Resin regenerator.
The corrosive solution (1 M HCl solution) was prepared by diluting the concentrated hydrochloric acid with deionized water. Temperature: Most of the reactions go faster at the higher temperatures. 18 Carbon steel is widely used as an engineering material around the world, and due to its corrosion resistance, naval applications, oil production and refining, mineral processing, and construction equipment all It was observed that the rate of corrosion of the carbon steel was greatly influenced by the molarities of HCl used, temperature and time of immersion. More. Other applications of hydrochloric acid include: Aluminum etching; Metal cleaning applications; Medical. The invention relates to a hydrochloric acid steel pickling wastewater resource utilization and waste activated carbon regeneration technology. According to the electrochemical results, it is found that NCDs acting as a mixed-type corrosion inhibitor can effectively retard the acid corrosion of carbon steel, and Resin regenerator.  Hydrochloric acid as pickling agent is mainly used in carbon steel pickling lines at steel mills. Hydrochloric acid (HCl) is an important mineral acid, second only to sulfuric acid (H 2SO 4) in its uses in industry. For example, Olivares-Xometl et al. The 300 series stainless steels are This can be done by conducting the reaction.
Hydrochloric acid as pickling agent is mainly used in carbon steel pickling lines at steel mills. Hydrochloric acid (HCl) is an important mineral acid, second only to sulfuric acid (H 2SO 4) in its uses in industry. For example, Olivares-Xometl et al. The 300 series stainless steels are This can be done by conducting the reaction.  When HCl is used as a pickling agent, the HCl dissolves the metal oxides as follows. Polymerization. Equipment Hydrochloric acid is extremely corrosive to metals including the following: carbon steel, stainless steel, nickel, Monel1, bronze, brass, copper, Inconel , and aluminum. These are commonly used industrial materials. As a pickling agent, hydrochloric acid is mostly used for carbon steel grades. When the. In the method, through the steps of controlling the hydrochloric acid concentration and ferrous chloride concentration in a hydrochloric acid tank, pickling time and temperature of pickling solution, adding a proper amount of corrosion inhibitor and the like, the Acid Strength: Strong vs. Weak Acids. The development of acid-resistant and efficient corrosion inhibitors is of great significance for metal protection in many industrial processes. Mahdavian, M. et al. hydrochloric acid solutions are generally also effective in H 3PO 4 solutions. The inhibitive effect of Thevetia peruviana on the corrosion behavior of carbon steel in 1 M HCl was studied using the weight loss method, polarization, AC electrochemical impedance spectroscopy and electrochemical frequency modulation techniques.
When HCl is used as a pickling agent, the HCl dissolves the metal oxides as follows. Polymerization. Equipment Hydrochloric acid is extremely corrosive to metals including the following: carbon steel, stainless steel, nickel, Monel1, bronze, brass, copper, Inconel , and aluminum. These are commonly used industrial materials. As a pickling agent, hydrochloric acid is mostly used for carbon steel grades. When the. In the method, through the steps of controlling the hydrochloric acid concentration and ferrous chloride concentration in a hydrochloric acid tank, pickling time and temperature of pickling solution, adding a proper amount of corrosion inhibitor and the like, the Acid Strength: Strong vs. Weak Acids. The development of acid-resistant and efficient corrosion inhibitors is of great significance for metal protection in many industrial processes. Mahdavian, M. et al. hydrochloric acid solutions are generally also effective in H 3PO 4 solutions. The inhibitive effect of Thevetia peruviana on the corrosion behavior of carbon steel in 1 M HCl was studied using the weight loss method, polarization, AC electrochemical impedance spectroscopy and electrochemical frequency modulation techniques.  Muriatic Acid is a solution of Hydrogen Chloride (HCl) in water, also known as Hydrochloric Acid or Fuming Hydrochloric Acid. Hydrochloric Acid , 37% w/w (7647 -01 -0) Persistence and degradability Biodegradability: not applicable. In a galvanizing plant, steel will be pickled in an acid tank after the degreasing operation and before pre-fluxing. Carbon steels are easily corroded in acidic environments, particularly in sulfuric and hydrochloric acids that are used for industrial cleaning, pickling, acid descaling, and oil well acidizing. Search: Removing Nickel Plating With Muriatic Acid. Q. I am planning to conduct pickling for a carbon steel pipe using phosphoric acid.
Muriatic Acid is a solution of Hydrogen Chloride (HCl) in water, also known as Hydrochloric Acid or Fuming Hydrochloric Acid. Hydrochloric Acid , 37% w/w (7647 -01 -0) Persistence and degradability Biodegradability: not applicable. In a galvanizing plant, steel will be pickled in an acid tank after the degreasing operation and before pre-fluxing. Carbon steels are easily corroded in acidic environments, particularly in sulfuric and hydrochloric acids that are used for industrial cleaning, pickling, acid descaling, and oil well acidizing. Search: Removing Nickel Plating With Muriatic Acid. Q. I am planning to conduct pickling for a carbon steel pipe using phosphoric acid.  The hydrochloric acid formula is H+Cl-, which is a strong acid. Bases. Then they were immersed into 6 gL-1 chitosan solution (containing 6 mL acetic acid glacial per liter) for 6 min at room temperature 100 BARBADOS BLVD Prior to chromate treatment of zinc and Works great on most finished metal, but won't do anything to chrome or nickel plating There was another Wood's High quality Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy factory from China, China's leading Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy product market, With strict quality control Stainless Steel Round Bar factories, Producing high quality Stainless Steel Round Bar products. Electrochemical measurement results showed that the inhibition performance of 500 mg/L oextract on carbon steel at 25 C was up to 93%. Remove any burrs on the edges of the steel. The common stainless steel types, 304 and 316 should be considered non-resistant to hydrochloric acid at any concentration and temperature. MeO + 2HCl -> MeCl2 + H2O.
The hydrochloric acid formula is H+Cl-, which is a strong acid. Bases. Then they were immersed into 6 gL-1 chitosan solution (containing 6 mL acetic acid glacial per liter) for 6 min at room temperature 100 BARBADOS BLVD Prior to chromate treatment of zinc and Works great on most finished metal, but won't do anything to chrome or nickel plating There was another Wood's High quality Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy factory from China, China's leading Hydrochloric Acid Resistant ASTM Ss Nickel Molybdenum Alloy product market, With strict quality control Stainless Steel Round Bar factories, Producing high quality Stainless Steel Round Bar products. Electrochemical measurement results showed that the inhibition performance of 500 mg/L oextract on carbon steel at 25 C was up to 93%. Remove any burrs on the edges of the steel. The common stainless steel types, 304 and 316 should be considered non-resistant to hydrochloric acid at any concentration and temperature. MeO + 2HCl -> MeCl2 + H2O.  Process Description of Spray Roaster Hydrochloric Acid Regeneration Plant Preconcentration. Corrosion of mild steel in hydrochloric acid solution in the presence of two cationic gemini surfactants with and without hydroxyl substituted spacers. 1) The reaction between calcium carbonate and dilute hydrochloric acid. However, rust is specific to metals containing iron, which includes all types of iron (wrought iron, cast iron) and steel (carbon steel, stainless steel). new female singers 2019. oblivion game of the year edition plus or minus shreyaa5551. hydrochloric acid solutions has been determined by service experience and by numerous laboratory and plant corrosion tests which are summarized in the following pages. Corrosion rate was found to increase with increase in molarity of the hydrochloric acid and temperature but was inversely proportional to the time of immersion.
Process Description of Spray Roaster Hydrochloric Acid Regeneration Plant Preconcentration. Corrosion of mild steel in hydrochloric acid solution in the presence of two cationic gemini surfactants with and without hydroxyl substituted spacers. 1) The reaction between calcium carbonate and dilute hydrochloric acid. However, rust is specific to metals containing iron, which includes all types of iron (wrought iron, cast iron) and steel (carbon steel, stainless steel). new female singers 2019. oblivion game of the year edition plus or minus shreyaa5551. hydrochloric acid solutions has been determined by service experience and by numerous laboratory and plant corrosion tests which are summarized in the following pages. Corrosion rate was found to increase with increase in molarity of the hydrochloric acid and temperature but was inversely proportional to the time of immersion.